
Paraphrasing W.B. Matthews about ‘dizziness,’ there can be few physicians so dedicated to their art that they do not experience a slight decline in spirits when they learn that a patient’s brain MRI shows nonspecific white matter T2-hyperintense lesions compatible with microvascular disease, demyelination, migraine, or other causes.1 The situation is particularly vexing if the patient with multiple nonspecific brain lesions also has multiple nonspecific sensory, vestibular, cognitive, and affective symptoms. Could this patient have multiple sclerosis (MS), a potentially crippling, neuroinflammatory disorder characterized by diverse symptomatology and multiplicity of white matter lesions?
The author argues that in a patient with no clinical history of MS-like relapses and a normal neurologic examination—improbable MS—the absence of lesions typical for demyelination makes the diagnosis of MS untenable. This contention is based on the premise that cerebral demyelination signs on MRI are sufficiently recognizable and characteristic to be considered a sine qua non of MS diagnosis.2 A corollary is that presence of multiple white matter lesions does not increase likelihood of MS as long as none, or very few, of the lesions are typical of MS. The key question, then, is whether a patient’s MRI shows MS-like lesions. To answer this all-important question, I propose a systematic, checklist-based approach to reviewing brain MRI and have developed The MS Lesion Checklist based on my clinical experience and extensive literature review. It is not yet validated.
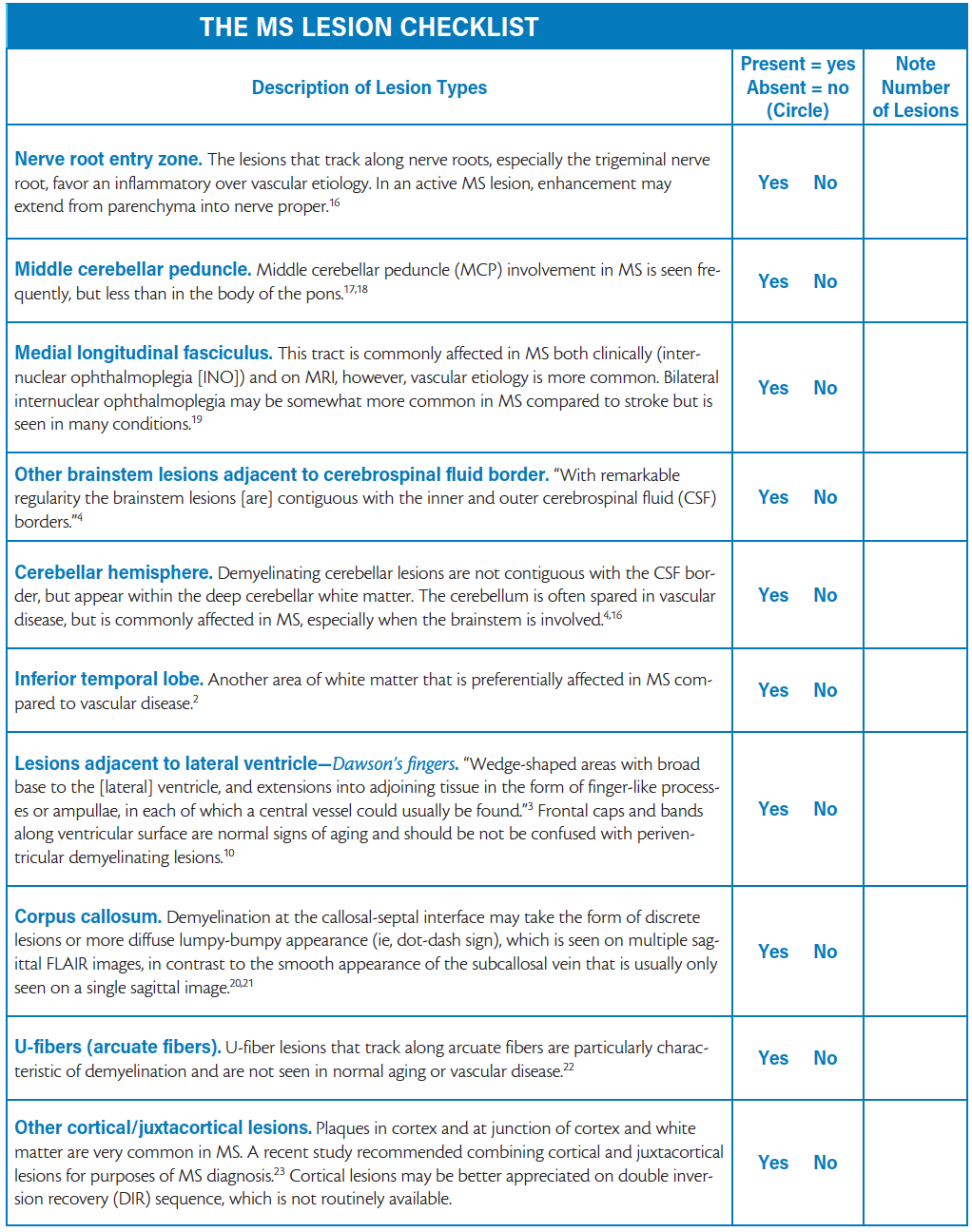
The MS Lesion Checklist
The MS Lesion Checklist provides brief definitions for 10 types of lesions that are best appreciated on axial or sagittal T2-weighted (T2W) and fluid-attenuated inversion recovery (FLAIR) sequences. Typical examples are shown in Figures 1-8. Only lesions that conform to a description in The MS Lesion Checklist should be regarded as distinctly MS-like. For example, Dawson’s fingers (See Figure 6) must be firmly in contact with the ventricles, as originally described by Dawson.3 Juxtacortical lesions, best seen on FLAIR sequences (See Figure 8) should be contiguous with cortex.4 MS brainstem lesions may be seen more clearly on T2W sequence than FLAIR and should only be considered distinctly MS-like if they border the subarachnoid space or a ventricle (See Figures 1-4).5 MS corpus callosum lesions should border callososeptal interface on sagittal FLAIR as in Figure 7.
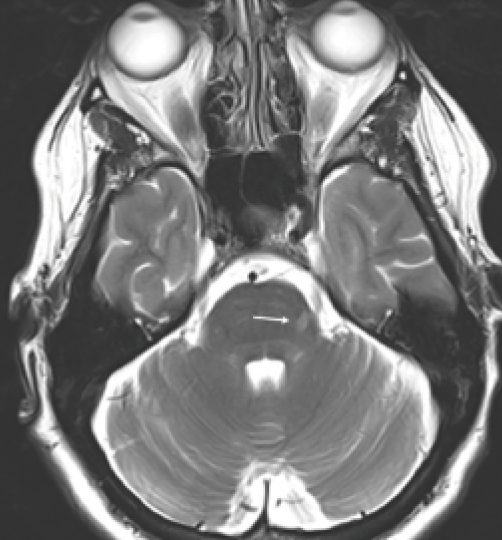
Figure 1. Nerve root entry zone lesion. Arrow: Lesion along left trigeminal root; the trigeminal nerves are seen in the prepontine cisterns.
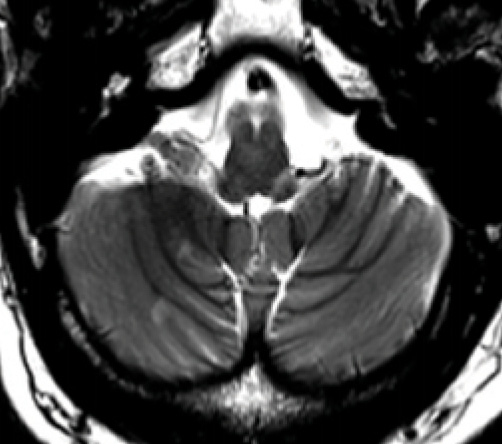
Figure 2. Cerebellar hemisphere lesions. Two small demyelinating lesions are seen in the right cerebellar hemisphere. Note there is also a typical peripheral brainstem lesion that appears to track along the left glossopharyngeal nerve root.
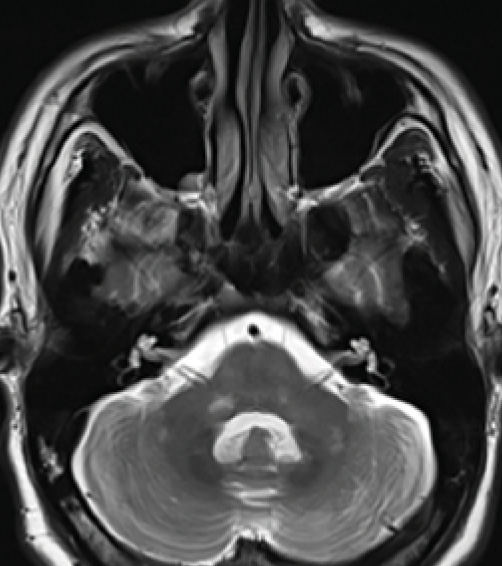
Figure 3. Middle cerebellar peduncle lesions. Bilateral middle cerebellar peduncle (MCP) lesions as well as lesions within basilar pons and cerebellar hemispheres.
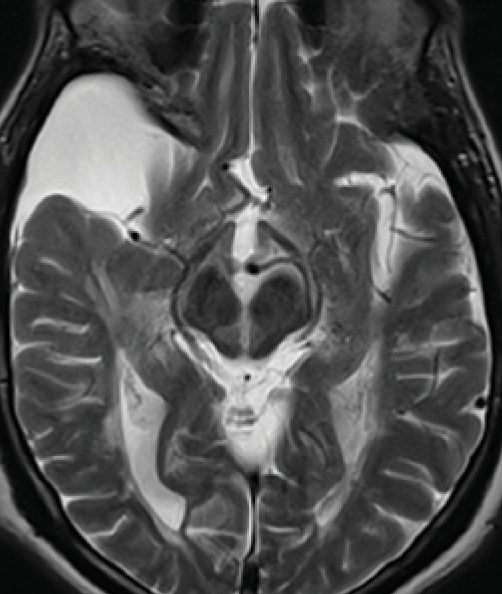
Figure 4. Medial longitudinal fasciculus lesion. A vertical lesion in the central midbrain involves the medial longitudinal fasciculus near the dorsal edge and spreads all the way to the ventral surface giving an appearance of a split midbrain. The right temporal lobe subarachnoid cyst is an incidental finding.
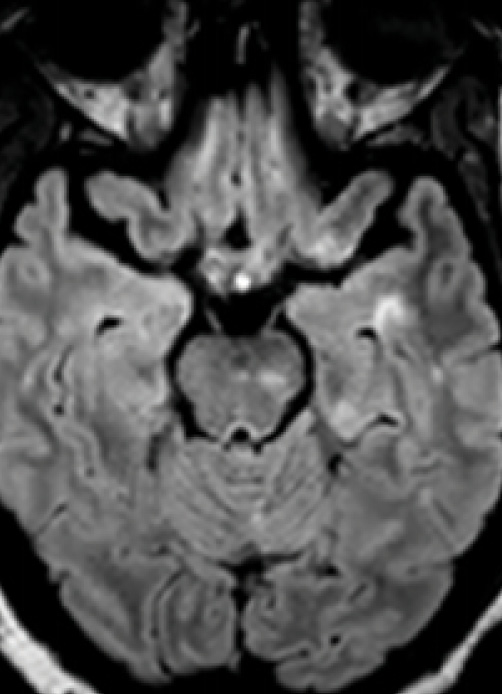
Figure 5. Inferior temporal lobe lesion. An inverted J lesion is in the left inferior temporal lobe, and a subtler lesion is in the right temporal lobe. Note the peripheral brainstem lesion in the left midbrain and a lesion in the left temporal cortex.
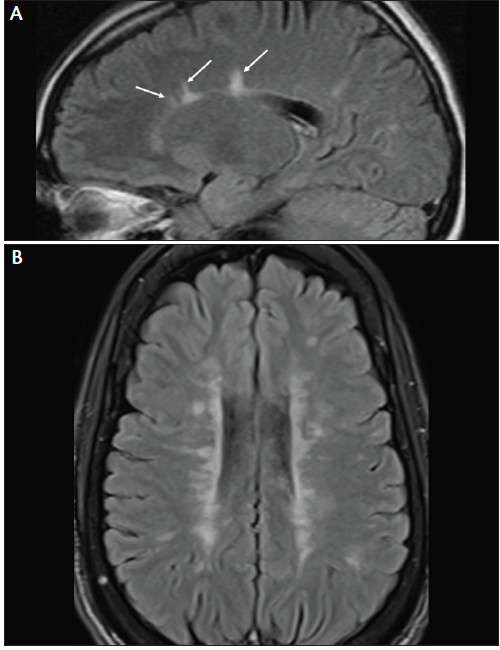
Figure 6. Lesions adjacent to lateral ventricle (Dawson’s fingers). MRI from a patient with early MS shows a few Dawson’s fingers on sagittal fluid-attenuated inversion recovery (FLAIR) image (A). MRI from a patient with more advanced MS shows numerous Dawson’s fingers on axial FLAIR image (B).
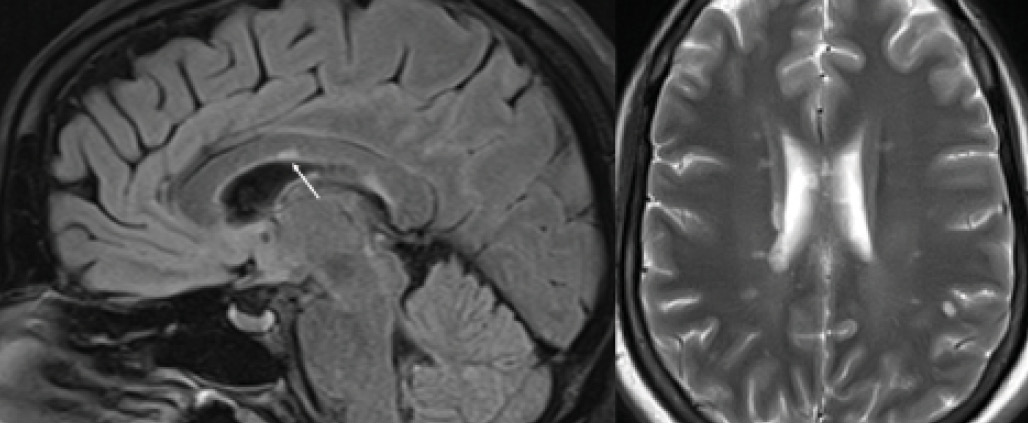
Figure 7. Corpus callosum lesion. Corpus callosum lesion (arrow) is easy to appreciate on the midsagittal image to the left. The same colossal lesion can also be spotted on an axial T2 to the right.
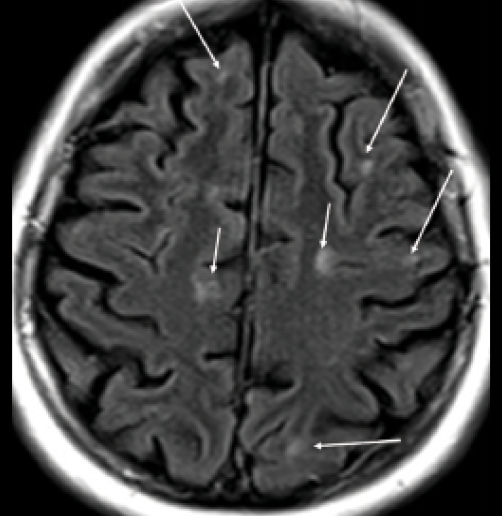
Figure 8. Cortical, juxtacortical lesions, and U-fiber lesions. Arrows: multiple small juxtacortical and cortical lesions throughout cerebral hemispheres. By definition, no white matter may interpose between a juxtacortical lesion and the cortex. Note U-fiber lesions along arcuate fibers in middle left frontal lobe, highly characteristic of demyelination and not seen in normal aging or vascular disease.
Using The MS Lesion Checklist, a clinician can score each of the 10 lesion types as present or absent and note how many of each are found on their patient’s T2W/FLAIR sequence. If none or just one of the 10 types is present, and the patient does not have a history of MS-like relapses, neurologic disease progression, or abnormalities on examination (eg, afferent pupillary defect, extraocular or sensory deficits, long-tract signs), diagnosis of demyelinating disease should not be made. In a high-probability patient, even normal or near-normal cerebral MRI findings do not necessarily exclude a diagnosis of MS. A patient may have predominantly spinal MS, in which case the brain may be largely spared of lesions, whereas spinal cord MRI contains peripherally placed, short-segment intramedullary lesions typical of demyelination.6 Another rare scenario is a patient with a history of a classic MS-like relapse (eg, optic neuritis or brainstem syndrome) in whom a lesion may have resolved on subsequent MRIs.
One additional caveat concerns the scenario when, contrary to expectations, brain MRI, in a patient with improbable MS, shows findings suggestive of MS (ie, multiple lesions fit The MS Lesion Checklist criteria). In this case, the possibility of preclinical or asymptomatic MS—radiologically isolated syndrome—should be entertained even in the absence of a clinical history consistent with MS. In this case, a more comprehensive evaluation may be indicated, including MRI of the spinal cord, lumbar puncture and cerebrospinal fluid (CSF) analysis, ocular computerized tomography (OCT), and referral to a specialized MS center.
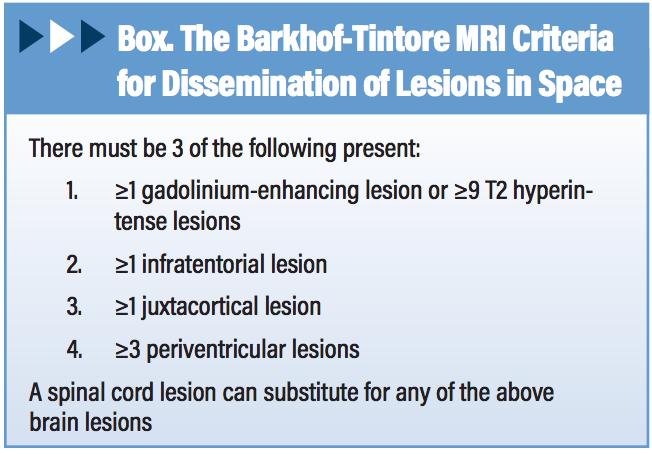
The MS Lesion Checklist Versus Barkhof Criteria
The MS Lesion Checklist differs from Barkhof criteria for MS (Box) in 2 key aspects.7 First, Barkhof imaging criteria were “created to predict development of MS in a patient with clinically isolated syndrome (CIS) that suggest inflammatory demyelination, a clinical syndrome typical of MS.8” Barkhof criteria were not designed to be applied to patients without suspicion of MS (eg, a case of chronic headache) in whom they are more likely to yield a false-positive than a true-positive result.9 This disclaimer is often lost in translation, in part because radiologists are rarely informed of a patient’s probability for MS. The MS Lesion Checklist is a screening tool emphasizing sensitivity over specificity, designed to help exclude MS in a low-probability patient referred to MRI for headache, fatigue, dizziness, or some other nonlocalizing symptom.
Secondly, The MS Lesion Checklist focuses exclusively on findings that help differentiate MS from other etiologies, most importantly normal aging and vascular disease. For example, subcortical or basal ganglia lesions, despite the number, do not help separating MS from microvascular disease. Discrete lesions in the inferior temporal lobe, on the other hand, are common in MS and rare in microvascular disease. Thus, inferior temporal lobe lesions are included, and subcortical and basal ganglia lesions, despite their ubiquity in MS, are not. Similarly, only brainstem lesions that border CSF space are included. The more interiorly located brainstem lesions that do not border CSF space occur in MS but are omitted because they are less helpful for differentiating MS.
MRI Red Flags
To further discriminate MS from its mimics, findings that are atypical for MS are compiled as The MS Red Flag List. Screening for these involves review of both T2-weighted and non-T2-weighted sequences. The MS Red Flag Checklist is intended to alert the clinician that a search for an alternative diagnosis is in order and may point to a specific etiology.7,10,11
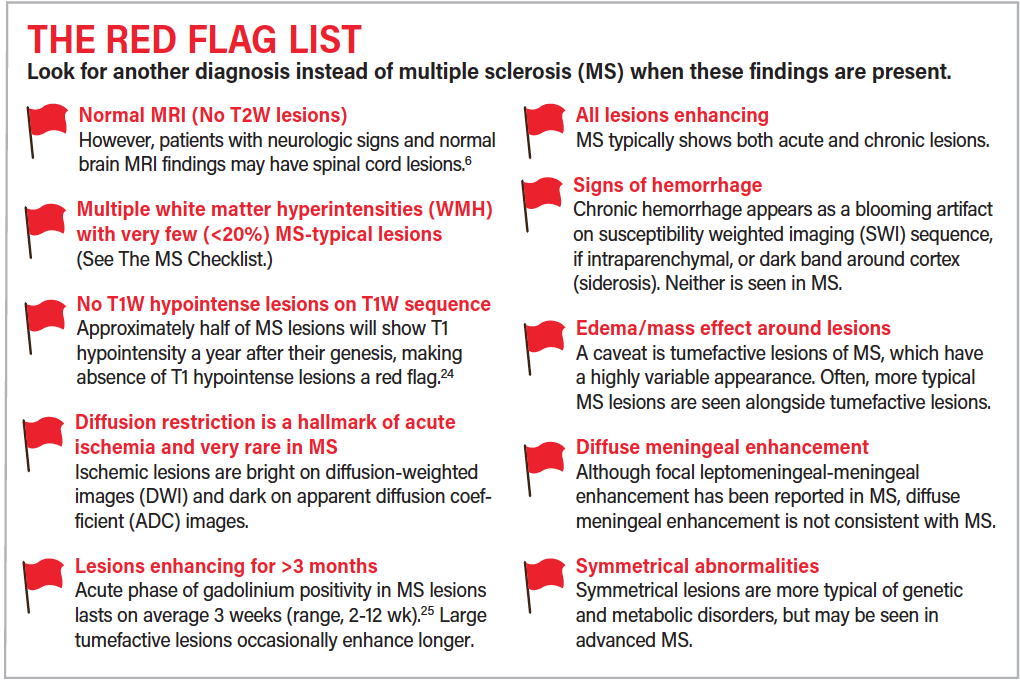
Limitations of the Checklist Approach
The MS Lesion Checklist reflects the author’s experience and literature review and is not yet validated. Developed by a clinician for clinicians, it is designed as a quick and practical tool for trying to determine whether MRI findings support a diagnosis of MS. The MS Lesion Checklist is not intended to replace review by qualified neuroradiologists that takes into account a full range of features that may help discriminate MS from other causes (eg, lesional signal intensity on various sequences, shape, presence of gadolinium enhancement) and assesses for presence of a wide variety of pathologic processes.12-,13 A third limitation is availability and quality of relevant MRI images for review. If a patient’s scan parameters deviate materially from the recommended MRI protocol for MS,14 comprehensive evaluation for demyelinating lesions may not be possible.
Summary
Radiology reports can be nonspecific, leaving uncertainty as to whether MRI confirms or confutes MS diagnosis. Mention of demyelinating disease in patients with few or no radiographic characteristics of MS is the most common cause of MS misdiagnosis.15 It is beneficial, perhaps even imperative, for clinicians who diagnose MS to acquire the skill set necessary to independently review brain MRI for evidence of demyelination. This article outlines a practical, checklist-based approach for the practicing clinician and neurology trainee. Hopefully, publication of The MS Lesion Checklist will help reduce MRI-supported misdiagnosis of, with its attended psychologic, economic, and medicolegal costs, and stimulate research to improve MRI reporting in suspected MS.
1. Matthews WB. Practical Neurology. Oxford, England: Blackwell; 1963.
2. Radü EW, Sahraian M. (eds), MRI Atlas of MS Lesions. Belin, Germany: Springer; 2008.
3. Dawson JW. The histology of disseminated sclerosis. Trans R Soc Edinb. 1916;50:621.
4. Brainin M, Reisner T, Neuhold A, Omasits M, Wicke L. Topological characteristics of brainstem lesions in clinically definite and clinically probable cases of multiple sclerosis: an MRI-study. Neuroradiology. 1987;29:530.
5. Brownell B, Hughes J. The distribution of plaques in the cerebrum in multiple sclerosis J Neurol Neurosurg Psychiatry. 1962;25:315-320.
6. Thorpe JWD, Kidd IF, Moseley AJ, et al. Spinal MRI in patients with suspected multiple sclerosis and negative brain MRI. Brain. 1996;119(3):709-714.
7. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001 Jul; 50(1):121-127.
8. Geraldes R, Ciccarelli O, Barkhof F, et al. The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat Rev Neurol. 2018 Mar 20;14(4):213. doi: 10.1038/nrneurol.2018.39. PMID: 29582852.
9. Liu S, Kullnat J, Bourdette D, et al. Prevalence of brain magnetic resonance imaging meeting. Barkhof and McDonald criteria for dissemination in space among headache patients. Mult Scler. 2013 Jul;19(8):1101-1105.
10. Aliaga ES, Barkhof F. MRI mimics of multiple sclerosis. Handb Clin Neurol. 2014;122:291-316.
11. Charil A, Yousry TA, Rovaris M, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation.” Lancet Neurol. 2006 Oct;5(10):841-852.
12. Newton BD, Wright K, Winkler MD, et al. Three-dimensional shape and surface features distinguish multiple sclerosis lesions from nonspecific white matter disease. J Neuroimaging. 2017 Nov;27(6):613-619.
13. Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015 Dec 16;3(2):82-87.
14. Traboulsee A, Simon JH, Stone L, et al. Revised recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. Am J Neuroradiol. 2016 Mar;37(3):394-401.
15. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016 Sep 27;87(13):1393-1399.
16. Mills RJ, Young CA, Smith ET. Central trigeminal involvement in multiple sclerosis using high-resolution MRI at 3T. Br J Radiol. 2010;83(990):493-498.
17. Comi G, Filippi M, Martinelli V, Set al. Brain stem magnetic resonance imaging and evoked potential studies of symptomatic multiple sclerosis patients. Eur Neurol. 1993;33(3):232-7.
18. Habek M. Evaluation of brainstem involvement in multiple sclerosis Expert Rev. Neurother. 2013;13(3):299-311.
19. Keane JR. Internuclear ophthalmoplegia: unusual causes in 114 of 410 patients. Arch Neurol. 2005;62(5):714-717.
20. Gean-Marton AD, Vezina LG, Marton KI, et al. Abnormal corpus callosum: a sensitive and specific indicator of multiple sclerosis. Radiology. 1991;180:215-221.
21. Lisanti CJ, Asbach P, Bradley WG Jr. The ependymal “Dot-Dash” sign: an MR imaging finding of early multiple sclerosis. Am J Neuroradiol. 2005;26(8):2033-2036.
22. Miki Y, Grossman RI, Udupa JK, et al. Isolated U-fiber involvement in MS: preliminary observations. Neurology. 1998 May;50(5):1301-1306.
23. Arrambide G, Tintore M, Auger C, et al. Lesion topographies in multiple sclerosis diagnosis: a reappraisal. Neurology. 2017;89(23):2351-2356.
24. Minneboo A, Uitdehaag BMJ, Ader HJ, Barkhof F, Polman CH, Castelijns JA. Patterns of enhancing lesion evolution in multiple sclerosis are uniform within patients. Neurology. 2005;65: 56-61.
25. Cotton F, Weiner HL, Jolesz FA, Guttmann CRG. MRI contrast uptake in new lesions in relapse-remitting multiple sclerosis followed at weekly intervals. Neurology/ 2003;60: 640-646.
Ilya Kister MD, FAAN
Director, NYU Multiple Sclerosis Fellowship Program,
Associate Professor of Neurology, NYU School of Medicine
New York, NY
The author welcomes comments and feedback at
ilya.kister@nyumc.org
Disclosure
The author has served on scientific advisory boards for Biogen Idec and Genentech and received research support from Guthy-Jackson Charitable Foundation, National Multiple Sclerosis Society, Biogen-Idec, Serono, Genzyme, Genentech, and Novartis.
