Abstract
Purpose
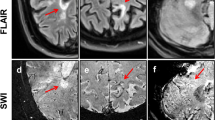
In multiple sclerosis (MS), chronic active/smoldering white matter lesions presenting with hypointense rims on susceptibility-weighted imaging (SWI) of the brain have been recognized as an important radiological feature. The aim of this work was to study the prevalence of paramagnetic rim lesions (RLs) in MS patients in a clinical setting and to assess differences in demographic and clinical variables regarding the presence of RLs.
Methods
All 3 T brain magnetic resonance (MR) studies performed in MS patients between July 2020 and January 2021 were reviewed. In all patients, RLs were assessed on three-dimensional (3D) SWI images and the T2 FLAIR lesion load volume was assessed. Demographic, laboratory (oligoclonal bands in CSF), and clinical data, including functional status with Expanded Disability Status Scale (EDSS), were retrieved from the clinical files.
Results
Of the 192 patients, 113 (59%) presented with at least 1 RL. In the RL-positive group, the mean RL count was 4.81 ranging from 1 to 37. There was no significant difference in the number of RLs between the different types of MS (p = 0.858). Regarding the presence of RLs, there were no significant differences based on gender (p = 0.083), disease duration (p = 0.520), treatment regime (p = 0.326), EDSS score (p = 0.103), and the associated T2 FLAIR lesion load volume.
Conclusion
SWI RLs were frequently detected in our cohort regardless of the MS type, T2 FLAIR lesion load volume, demographic features, disease duration, or clinical score. Our results suggest that RLs are not associated with more severe forms of the disease. Today, RLs can be seen on 3 T 3D SWI, although this is not a clinical standard sequence yet. Therefore, it should be considered an additional helpful MR sequence in the diagnostic workup of MS, although more studies are warranted to establish the role of RLs as prognostic markers.





Similar content being viewed by others
Data availability
All data is available under request.
Code availability
Not applicable.
References
Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, Rocca MA (2018) Multiple sclerosis. Nat Rev Dis Primers 4:43. https://doi.org/10.1038/s41572-018-0041-4
McGinley MP, Goldschmidt CH, Rae-Grant AD (2021) Diagnosis and treatment of multiple sclerosis: a review. JAMA 325:765–779. https://doi.org/10.1001/jama.2020.26858
Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I, Mandrekar J, Bramow S, Metz I, Bruck W, Lassmann H, Lucchinetti CF (2015) Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 78:710–721. https://doi.org/10.1002/ana.24497
Absinta M, Sati P, Masuzzo F, Nair G, Sethi V, Kolb H, Ohayon J, Wu T, Cortese ICM, Reich DS (2019) Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol 76:1474–1483. https://doi.org/10.1001/jamaneurol.2019.2399
Chawla S, Kister I, Wuerfel J, Brisset JC, Liu S, Sinnecker T, Dusek P, Haacke EM, Paul F, Ge Y (2016) Iron and non-iron-related characteristics of multiple sclerosis and neuromyelitis optica lesions at 7T MRI. AJNR Am J Neuroradiol 37:1223–1230. https://doi.org/10.3174/ajnr.A4729
Clarke MA, Samaraweera AP, Falah Y, Pitiot A, Allen CM, Dineen RA, Tench CR, Morgan PS, Evangelou N (2020) Single Test to ARrive at Multiple Sclerosis (STAR-MS) diagnosis: a prospective pilot study assessing the accuracy of the central vein sign in predicting multiple sclerosis in cases of diagnostic uncertainty. Mult Scler 26:433–441. https://doi.org/10.1177/1352458519882282
Dal-Bianco A, Grabner G, Kronnerwetter C, Weber M, Hoftberger R, Berger T, Auff E, Leutmezer F, Trattnig S, Lassmann H, Bagnato F, Hametner S (2017) Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol 133:25–42. https://doi.org/10.1007/s00401-016-1636-z
Harrison DM, Li X, Liu H, Jones CK, Caffo B, Calabresi PA, van Zijl P (2016) Lesion heterogeneity on high-field susceptibility MRI is associated with multiple sclerosis severity. AJNR Am J Neuroradiol 37:1447–1453. https://doi.org/10.3174/ajnr.A4726
Absinta M, Sati P, Gaitan MI, Maggi P, Cortese IC, Filippi M, Reich DS (2013) Seven-tesla phase imaging of acute multiple sclerosis lesions: a new window into the inflammatory process. Ann Neurol 74:669–678. https://doi.org/10.1002/ana.23959
Filippi M, Preziosa P, Banwell BL, Barkhof F, Ciccarelli O, De Stefano N, Geurts JJG, Paul F, Reich DS, Toosy AT, Traboulsee A, Wattjes MP, Yousry TA, Gass A, Lubetzki C, Weinshenker BG, Rocca MA (2019) Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain 142:1858–1875. https://doi.org/10.1093/brain/awz144
Absinta M, Sati P, Schindler M, Leibovitch EC, Ohayon J, Wu T, Meani A, Filippi M, Jacobson S, Cortese IC, Reich DS (2016) Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 126:2597–2609. https://doi.org/10.1172/JCI86198
Bagnato F, Hametner S, Yao B, van Gelderen P, Merkle H, Cantor FK, Lassmann H, Duyn JH (2011) Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 134:3602–3615. https://doi.org/10.1093/brain/awr278
Mehta V, Pei W, Yang G, Li S, Swamy E, Boster A, Schmalbrock P, Pitt D (2013) Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. PLoS ONE 8:e57573. https://doi.org/10.1371/journal.pone.0057573
Yao B, Ikonomidou VN, Cantor FK, Ohayon JM, Duyn J, Bagnato F (2015) Heterogeneity of multiple sclerosis white matter lesions detected with T2*-weighted imaging at 7.0 Tesla. J Neuroimaging 25:799–806. https://doi.org/10.1111/jon.12193
Dal-Bianco A, Grabner G, Kronnerwetter C, Weber M, Kornek B, Kasprian G, Berger T, Leutmezer F, Rommer PS, Trattnig S, Lassmann H, Hametner S (2021) Long-term evolution of multiple sclerosis iron rim lesions in 7 T MRI. Brain. https://doi.org/10.1093/brain/awaa436
Popescu BF, Frischer JM, Webb SM, Tham M, Adiele RC, Robinson CA, Fitz-Gibbon PD, Weigand SD, Metz I, Nehzati S, George GN, Pickering IJ, Bruck W, Hametner S, Lassmann H, Parisi JE, Yong G, Lucchinetti CF (2017) Pathogenic implications of distinct patterns of iron and zinc in chronic MS lesions. Acta Neuropathol 134:45–64. https://doi.org/10.1007/s00401-017-1696-8
Correale J, Gaitan MI, Ysrraelit MC, Fiol MP (2017) Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain 140:527–546. https://doi.org/10.1093/brain/aww258
Elliott C, Belachew S, Wolinsky JS, Hauser SL, Kappos L, Barkhof F, Bernasconi C, Fecker J, Model F, Wei W, Arnold DL (2019) Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 142:2787–2799. https://doi.org/10.1093/brain/awz212
Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I (2018) Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol 135:511–528. https://doi.org/10.1007/s00401-018-1818-y
Hammond KE, Metcalf M, Carvajal L, Okuda DT, Srinivasan R, Vigneron D, Nelson SJ, Pelletier D (2008) Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol 64:707–713. https://doi.org/10.1002/ana.21582
Yao B, Bagnato F, Matsuura E, Merkle H, van Gelderen P, Cantor FK, Duyn JH (2012) Chronic multiple sclerosis lesions: characterization with high-field-strength MR imaging. Radiology 262:206–215. https://doi.org/10.1148/radiol.11110601
Bian W, Harter K, Hammond-Rosenbluth KE, Lupo JM, Xu D, Kelley DA, Vigneron DB, Nelson SJ, Pelletier D (2013) A serial in vivo 7T magnetic resonance phase imaging study of white matter lesions in multiple sclerosis. Mult Scler 19:69–75. https://doi.org/10.1177/1352458512447870
Maggi P, Sati P, Nair G, Cortese ICM, Jacobson S, Smith BR, Nath A, Ohayon J, van Pesch V, Perrotta G, Pot C, Theaudin M, Martinelli V, Scotti R, Wu T, Du Pasquier R, Calabresi PA, Filippi M, Reich DS, Absinta M (2020) Paramagnetic rim lesions are specific to multiple sclerosis: an international multicenter 3T MRI study. Ann Neurol 88:1034–1042. https://doi.org/10.1002/ana.25877
Calvi A, Haider L, Prados F, Tur C, Chard D, Barkhof F (2020) In vivo imaging of chronic active lesions in multiple sclerosis. Mult Scler 26:1–8. https://doi.org/10.1177/1352458520958589
Blindenbacher N, Brunner E, Asseyer S, Scheel M, Siebert N, Rasche L, Bellmann-Strobl J, Brandt A, Ruprecht K, Meier D, Wuerfel J, Paul F, Sinnecker T (2020) Evaluation of the “ring sign” and the “core sign” as a magnetic resonance imaging marker of disease activity and progression in clinically isolated syndrome and early multiple sclerosis. Mult Scler J Exp Transl Clin 6:2055217320915480. https://doi.org/10.1177/2055217320915480
Hagemeier J, Weinstock-Guttman B, Bergsland N, Heininen-Brown M, Carl E, Kennedy C, Magnano C, Hojnacki D, Dwyer MG, Zivadinov R (2012) Iron deposition on SWI-filtered phase in the subcortical deep gray matter of patients with clinically isolated syndrome may precede structure-specific atrophy. AJNR Am J Neuroradiol 33:1596–1601. https://doi.org/10.3174/ajnr.A3030
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintore M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Lorscheider J, Buzzard K, Jokubaitis V, Spelman T, Havrdova E, Horakova D, Trojano M, Izquierdo G, Girard M, Duquette P, Prat A, Lugaresi A, Grand’Maison F, Grammond P, Hupperts R, Alroughani R, Sola P, Boz C, Pucci E, Lechner-Scott J, Bergamaschi R, Oreja-Guevara C, Iuliano G, Van Pesch V, Granella F, Ramo-Tello C, Spitaleri D, Petersen T, Slee M, Verheul F, Ampapa R, Amato MP, McCombe P, Vucic S, Sánchez Menoyo JL, Cristiano E, Barnett MH, Hodgkinson S, Olascoaga J, Saladino ML, Gray O, Shaw C, Moore F, Butzkueven H, Kalincik T, MSBase Study Group (2016) Defining secondary progressive multiple sclerosis. Brain 139:2395–2405. https://doi.org/10.1093/brain/aww173
Okuda DT, Siva A, Kantarci O, Inglese M, Katz I, Tutuncu M, Keegan BM, Donlon S, Hua LH, Vidal-Jordana A, Montalban X, Rovira A, Tintoré M, Amato MP, Brochet B, de Seze J, Brassat D, Vermersch P, De Stefano N, Sormani MP, Pelletier D, Lebrun C, Radiologically Isolated Syndrome C, Club Francophone de la Sclérose en Plaques (2014) Radiologically isolated syndrome: 5-year risk for an initial clinical event. PloS One 9:e90509–e90509. https://doi.org/10.1371/journal.pone.0090509
Philips (2019) IntelliSpace Portal 9 LOBI COBI. 8
Chawla S, Kister I, Sinnecker T, Wuerfel J, Brisset JC, Paul F, Ge Y (2018) Longitudinal study of multiple sclerosis lesions using ultra-high field (7T) multiparametric MR imaging. PLoS ONE 13:e0202918. https://doi.org/10.1371/journal.pone.0202918
Clarke MA, Pareto D, Pessini-Ferreira L, Arrambide G, Alberich M, Crescenzo F, Cappelle S, Tintore M, Sastre-Garriga J, Auger C, Montalban X, Evangelou N, Rovira A (2020) Value of 3T susceptibility-weighted imaging in the diagnosis of multiple sclerosis. AJNR Am J Neuroradiol 41:1001–1008. https://doi.org/10.3174/ajnr.A6547
Kaunzner UW, Kang Y, Zhang S, Morris E, Yao Y, Pandya S, Hurtado Rua SM, Park C, Gillen KM, Nguyen TD, Wang Y, Pitt D, Gauthier SA (2019) Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain 142:133–145. https://doi.org/10.1093/brain/awy296
Prineas JW, Kwon EE, Cho ES, Sharer LR, Barnett MH, Oleszak EL, Hoffman B, Morgan BP (2001) Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol 50:646–657. https://doi.org/10.1002/ana.1255
Chen W, Gauthier SA, Gupta A, Comunale J, Liu T, Wang S, Pei M, Pitt D, Wang Y (2014) Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. Radiology 271:183–192. https://doi.org/10.1148/radiol.13130353
Voet S, Prinz M, van Loo G (2019) Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol Med 25:112–123. https://doi.org/10.1016/j.molmed.2018.11.005
al TAe (2021) Efficacy and safety of tolebrutinib in patients with highly active relapsing MS: subgroup analysis of the phase 2b study. AAN 2021
Filippi M, Bruck W, Chard D, Fazekas F, Geurts JJG, Enzinger C, Hametner S, Kuhlmann T, Preziosa P, Rovira A, Schmierer K, Stadelmann C, Rocca MA, Attendees of the Correlation between P, workshop MRIfiM (2019) Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol 18:198–210. https://doi.org/10.1016/S1474-4422(18)30451-4
Sethi V, Nair G, Absinta M, Sati P, Venkataraman A, Ohayon J, Wu T, Yang K, Shea C, Dewey BE, Cortese IC, Reich DS (2017) Slowly eroding lesions in multiple sclerosis. Mult Scler 23:464–472. https://doi.org/10.1177/1352458516655403
Eisele P, Fischer K, Szabo K, Platten M, Gass A (2019) Characterization of contrast-enhancing and non-contrast-enhancing multiple sclerosis lesions using susceptibility-weighted imaging. Front Neurol 10:1082. https://doi.org/10.3389/fneur.2019.01082
Sinnecker T, Clarke MA, Meier D, Enzinger C, Calabrese M, De Stefano N, Pitiot A, Giorgio A, Schoonheim MM, Paul F, Pawlak MA, Schmidt R, Kappos L, Montalban X, Rovira A, Evangelou N, Wuerfel J, Group MS (2019) Evaluation of the central vein sign as a diagnostic imaging biomarker in multiple sclerosis. JAMA Neurol 76:1446–1456. https://doi.org/10.1001/jamaneurol.2019.2478
Thompson AJ, Reingold SC, Cohen JA, International Panel on Diagnosis of Multiple Sclerosis (2018) Applying the 2017 McDonald diagnostic criteria for multiple sclerosis - Authors’ reply. Lancet Neurol 17:499–500. https://doi.org/10.1016/S1474-4422(18)30168-6
Wattjes MP, Ciccarelli O, Reich DS, Banwell B, de Stefano N, Enzinger C, Fazekas F, Filippi M, Frederiksen J, Gasperini C, Hacohen Y, Kappos L, Li DKB, Mankad K, Montalban X, Newsome SD, Oh J, Palace J, Rocca MA, Sastre-Garriga J, Tintoré M, Traboulsee A, Vrenken H, Yousry T, Barkhof F, Rovira À. (2021) 2021 MAGNIMS–CMSC–NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol https://doi.org/10.1016/S1474-4422(21)00095-8
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was approved by the local ethics committee (number 2811).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pinto, C., Cambron, M., Dobai, A. et al. Smoldering lesions in MS: if you like it then you should put a rim on it. Neuroradiology 64, 703–714 (2022). https://doi.org/10.1007/s00234-021-02800-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02800-0