Abstract
Almost all plant cells have large vacuoles that contain both hydrolytic enzymes and a variety of defense proteins. Plants use vacuoles and vacuolar contents for programmed cell death (PCD) in two different ways: for a destructive way and for a non-destructive way. Destruction is caused by vacuolar membrane collapse, followed by the release of vacuolar hydrolytic enzymes into the cytosol, resulting in rapid and direct cell death. The destructive way is effective in the digestion of viruses proliferating in the cytosol, in susceptible cell death induced by fungal toxins, and in developmental cell death to generate integuments (seed coats) and tracheary elements. On the other hand, the non-destructive way involves fusion of the vacuolar and the plasma membrane, which allows vacuolar defense proteins to be discharged into the extracellular space where the bacteria proliferate. Membrane fusion, which is normally suppressed, was triggered in a proteasome-dependent manner. Intriguingly, both ways use enzymes with caspase-like activity; the membrane-fusion system uses proteasome subunit PBA1 with caspase-3-like activity, and the vacuolar-collapse system uses vacuolar processing enzyme (VPE) with caspase-1-like activity. This review summarizes two different ways of vacuole-mediated PCD and discusses how plants use them to attack pathogens that invade unexpectedly.
Similar content being viewed by others
Plant Vacuoles as Lytic and Storage Compartments
Most mature plant cells have vacuoles that occupy a large part of the cell volume. This feature is unique to plant cells. There are two types of vacuoles, lytic vacuoles and protein storage vacuoles.1 Lytic vacuoles contain hydrolytic enzymes to degrade cellular materials that are no longer required, whereas protein storage vacuoles accumulate large amounts of various proteins such as defense proteins, and storage proteins for seed germination and subsequent seedling growth. As lytic vacuoles and protein storage vacuoles have opposite functions, degradation and storage, respectively, these two vacuoles have been distinguished using marker proteins such as tonoplast intrinsic proteins. However, recent studies suggest that both types of vacuoles share machinery for the intracellular trafficking of vacuolar proteins.2, 3 Most vacuolar soluble proteins are synthesized on the endoplasmic reticulum as larger precursors and then transported into vacuoles, where precursor proteins are converted into their respective mature forms by vacuolar processing enzyme (VPE).4, 5, 6, 7, 8 The machinery in plant cells is used to accumulate a variety of proteins in both types of vacuoles, hydrolytic enzymes including aspartate proteinases,9 cysteine proteinases,10, 11 and nucleases12 required for non-selective degradation of cellular components during programmed cell death (PCD), and defense proteins including pathogenesis-related proteins (PR proteins),13 myrosinases,14 toxic proteins,15 and lectins16, 17 for defense against invading pathogens.
Non-Destructive versus Destructive Vacuole-mediated Cell Death
As plants do not have mobile immune cells, they have evolved unique immune systems with different defense strategies for different pathogens.18 One of these strategies is the hypersensitive response (HR), which confers broad-spectrum disease resistance in plants. The HR is often accompanied by rapid and localized PCD, known as hypersensitive cell death, at the infection site to prevent the growth and spread of pathogens into healthy tissues.19, 20 This response is initiated by the direct or indirect recognition of a pathogen avirulence (Avr) factor by a plant resistance gene product and is controlled by multiple signal transduction pathways.21
Hypersensitive cell death triggered by some pathogens is caused by vacuole-mediated cell death, which is a type of plant-specific PCD. Using vacuoles for defense-related cell death makes sense for plants, because vacuoles exist in each cell of plants. The question is, how are vacuoles used for cell death? There are two different ways of vacuole-mediated cell death, a destructive type triggered by vacuolar membrane collapse22, 23, 24, 25, 26 and a non-destructive type involving no vacuolar membrane collapse27 (Figure 1). The non-destructive way, which commences with the vacuolar membrane and cytoplasm intact, were recently observed in avirulent bacteria-induced hypersensitive cell death.27 The cell death by non-destructive way is caused by membrane fusion of the vacuolar membrane and the plasma membrane (Figure 1, upper). Membrane fusion discharges vacuolar hydrolytic enzymes into the extracellular matrix, resulting in cell death. On the other hand, the destructive way is initiated by the collapse of the vacuolar membrane, which releases vacuolar hydrolytic enzymes directly into the cytosol to degrade cytoplasmic components, resulting in rapid and direct cell death (Figure 1, lower). They are effective for the digestion of viral pathogens proliferating in the cytosol,23 for susceptible cell death induced by fungal toxins,24 and for developmental cell death to generate integuments (seed coats)25 and tracheary elements.26
Two different ways of vacuole-mediated cell death: a destructive way triggered by vacuolar membrane collapse and a non-destructive way involving no vacuolar membrane collapse. The non-destructive way involves fusion between the vacuolar membrane and the plasma membrane leading to discharge of vacuolar hydrolytic enzymes outside of the cell, resulting in indirect cell death (upper). The destructive way is caused by vacuolar membrane collapse followed by the release of vacuolar hydrolytic enzymes into the cytosol, resulting in rapid and direct cell death (lower). V, vacuole; cw, cell wall; pm, plasma membrane
Membrane Fusion-mediated Cell Death without Destruction of Vacuolar Membrane
Non-destructive way
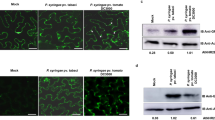
Arabidopsis thaliana plants are resistant to Pseudomonas syringae pv. tomato that have the Avr gene avrRpm1 (Pst DC3000/avrRpm1)28 and avrRpt2 (Pst DC3000/avrRpt2).29, 30 Leaf cells infected with Pst DC3000/avrRpm1 were intact at 3 h, but exhibited cell shrinkage and cytoplasmic aggregation, which are characteristic of hypersensitive cell death, at 12 h. Ultrastructural analysis of infected cells showed that the membrane of the large central vacuole is uniformly and frequently fused with the plasma membrane 3 h after infection (Figures 2b and c).27 The membrane fusion occurred nearly simultaneously in 83% of the cells examined at 3 h and in most of the cells at 6 h. Another avirulent strain, Pst DC3000/avrRpt2, caused membrane fusion at 7.5 h after inoculation. On the other hand, membrane fusion was not detected in cells of the rpm1 mutant even at 12 h after the inoculation of Pst DC3000/avrRpm1. Similarly, membrane fusion was not caused by the inoculation of Pst DC3000, which has neither avrRpm1 nor avrRpt2. Taken together, these results indicated that an interaction between RPM1 (a plant R gene product) and AvrRpm1 (a pathogen Avr factor) is required for membrane fusion.
Two types cell autonomous immune systems through vacuole-mediated cell death. Membrane fusion-mediated hypersensitive cell death against bacterial pathogens (a) and vacuolar collapse-mediated hypersensitive cell death against viral pathogens (b). Electron microscopic pictures show bacterial infection-induced membrane fusion (c and d) and viral infection-induced vacuolar membrane collapse (e). FS, a fusion suppressor; Ub, ubiquitin
The fusion resulted in the interconnection of vacuoles and the outside spaces of the plasma membrane in leaf cells, which made it possible to discharge vacuolar contents outside of the cells (Figure 2a). A vacuolar-localized fluorescent protein became detectable outside the cells after the inoculation of avirulent strains Pst DC3000/avrRpm1 and Pst DC3000/avrRpt2. Vacuolar proteolytic enzymes were detected in extracellular fluid from the leaves at 3 h after the inoculation of Pst DC3000/avrRpm1, and their levels increased at 4.5 h. Interestingly, extracellular fluid from infected leaves exhibited both antibacterial activity and cell death-induction activity.
Vacuolar defense proteins are known to accumulate after the infection of bacterial pathogens.31 How these proteins attack the bacteria, which proliferate in the extracellular space, has been a mystery. This issue was solved with the finding that fusion of the vacuolar plasma membrane allows vacuolar defense proteins to be discharged into the extracellular space where the bacteria proliferate. Formation of a tunnel from the inside to the outside of the cell wards off the bacteria.
Proteasome-dependent membrane fusion
Membrane fusion between the central vacuole and the plasma membrane does not occur under normal conditions. This leads to the hypothesis that a fusion suppressor exists in the cells. Bacterial infection could trigger degradation of this fusion suppressor, resulting in membrane fusion (Figure 2a). This hypothesis is supported by the finding that proteasome function is required for membrane fusion, followed by hypersensitive cell death in response to avirulent bacterial infection.27
The Arabidopsis proteasome has three catalytic subunits, PBA1, PBB, and PBE. Treatment with a PBA1 inhibitor (Ac-APnLD-CHO) suppressed not only membrane fusion, but also the discharge of vacuolar proteins outside the infected cells.27 Silencing each gene of PBA1, PBB, and PBE blocked membrane fusion after inoculation with avirulent bacteria (Pst DC3000/avrRpm1).27 All of the RNAi lines became sensitive to bacterial infection, suggesting that deficiency of one subunit causes a defect in proteasome function, and that proteasome function is required for disease resistance.27 This is consistent with suggestions on the involvement of ubiquitination in disease resistance.32, 33 Proteasome-dependent degradation of some factor might trigger membrane fusion, followed by the discharge of vacuolar contents for the purpose of attacking bacteria (Figure 2a).
The next question is whether the proteasome has a role in hypersensitive cell death induced by bacteria. Hypersensitive cell death can be monitored by either trypan-blue staining of dead cells, or by measuring ion leakage from dead cells. Results from both experiments reveal that deficiency of each proteasome subunit suppresses hypersensitive cell death in response to the infection of either Pst DC3000/avrRpm1 or Pst DC3000/avrRpt2, although it did not affect susceptible cell death caused by the virulent strain, Pst DC3000.27 PBA1 deficiency does not suppress the induction of the NADPH oxidases (AtrbohD and AtrbohF) responsible for generating reactive oxygen intermediates, or PR-1 and PR-2.27 Proteasome function confers cell-autonomous immunity to bacterial pathogens through hypersensitive cell death.
Involvement of caspase-3-like activity
PCD is a basic physiological process that occurs under various stresses and during the development in plants and animals, and some regulatory mechanisms underlying PCD are thought to be conserved in both organisms. Apoptotic cell death in animals is regulated by cysteine proteinases called caspases. Many studies have shown that activities similar to those of caspases are required for various types of cell death of plants.34, 35, 36 As plants lack genes homologous to caspases, the question is, what proteinases are responsible for caspase-like activity?
Previously, the vacuolar enzyme VPE was reported to be a proteinase that exhibits caspase-1-like activity (discussed below). However, neither VPE deficiency nor a caspase-1 inhibitor blocks bacterially-induced hypersensitive cell death. Instead, it is effectively blocked by a caspase-3 inhibitor, as described above. This indicates that the membrane fusion system for bacterially-induced cell death involves caspase-3-like activity. The proteinase responsible for caspase-3-like activity is found to be a proteasome β1 subunit, which is one of three catalytic subunits (β1, β2, and β5).37, 38
In Arabidopsis, the genes for these three subunits are PBA1, PBB, and PBE, respectively.39 Arabidopsis RNAi lines, in which the PBA1 gene was specifically suppressed, had reduced caspase-3-like activity (DEVDase), which is highly correlated with PBA1 activity. A pull-down analysis with biotin-DEVD-fmk, followed by an immunoblot with anti-PBA1 antibody showed that PBA1 is responsible for the DEVDase activity. This analysis unveiled PBA1 as a previously unidentified plant enzyme that has long been reported to function in hypersensitive cell death.
A model of membrane fusion-mediated cell death
Proteasome-dependent cell death involves three processes (Figure 2a). The first process concerns the fusion of a large central vacuole with the plasma membrane after bacterial infection. Bacterial infection may trigger ubiquitination of an unknown fusion suppressor in proteasome-dependent degradation.
The second process entails the discharge of vacuolar contents, including antibacterial proteins and hydrolytic enzymes, to the outside of the plasma membrane, as well as the production of defense proteins in the cell after membrane fusion. Topologically, membrane fusion between the vacuolar membrane and the plasma membrane does not damage the cytoplasm. Even after membrane fusion, cells remain alive and their ability to produce antibacterial materials is maintained for about 12 h after infection by Pst DC3000/avrRpm1, until the time of death.
In the third process, cells induce hypersensitive cell death by actions of hydrolytic enzymes released from vacuoles to the outside of the cell. Unlike animal pathogens, phytopathogenic bacteria do not enter host cells; instead, they proliferate in the intracellular spaces of the leaves. This novel defense strategy involving proteasome-regulating membrane fusion between the vacuolar and plasma membranes provides plants with a mechanism for attacking intercellular bacterial pathogens that invade through stomata on the leaves. The immune system complements another vacuolar defense mechanism in which viral propagation inside the cell is checked by vacuolar collapse, as described below.
VPE-Dependent Cell Death Through the Destruction of Vacuoles
Destruction of vacuoles
The second type of vacuole-mediated cell death is associated with the collapse of the vacuolar membrane, resulting in the release of vacuolar hydrolytic enzymes into the cytosol (Figure 1, lower). It is an efficient defense against viruses proliferating in the cytosol (Figure 2d). Tobacco mosaic virus induces typical visible lesions with shrinkage of cells in the leaves of Nicotiana benthamiana, in which the vacuolar membrane is disrupted23 (Figure 2e). The vacuolar enzymes, including nucleases and proteinases, easily degrade the virus composed of RNA and proteins.
The destruction of vacuoles also leads to direct cell death through the degradation of various organelles, including the nucleus. How is the nuclear DNA degraded? Most PCDs are accompanied by the fragmentation of nuclear DNA. Formation of a nucleosomal DNA ladder is known to be a typical feature of apoptosis.40 However, some plant PCDs do not involve DNA ladder formation.41, 42 Nuclear DNA of the TMV-infected leaves was cleaved into ∼50-kb fragments,23 which is inconsistent with the formation of a nucleosomal DNA ladder. The fact that vacuolar nucleases and proteinases are involved in the non-selective digestion of nucleosomes composed of DNA and histones, respectively, makes the formation of such a ladder unlikely. It is concluded that destructive cell death is not necessarily accompanied by the formation of a nucleosomal DNA ladder.
Virus-induced cell death is a HR involving rapid and localized cell death at infected regions to prevent infestation of pathogens.23 Plants lacking macrophages use vacuoles to degrade cellular materials and viruses through vacuolar destruction.
VPE-dependent hypersensitive cell death
Vacuole-mediated cell death through destruction is initiated by a vacuolar enzyme, VPE.22, 23, 43 VPE-gene silencing completely suppresses lesion formation in TMV-infected leaves, vacuolar collapse, and DNA fragmentation. Although VPE deficiency prevents these typical characteristics, it does not interfere with the production of defense proteins (PR proteins).23 This means that the process of vacuole-mediated cell death is independent of defense-protein production. Considering that VPE appears rapidly at the beginning of the TMV-induced HR and declines before forming visible lesions,23 VPE is essential in an early step of virus-induced cell death.
VPE-dependent cell death through destruction is effective in eliminating viruses within the cell. However, it is not effective in preventing bacteria from proliferating outside cells. Plants have evolved a cell-autonomous immune system based on membrane fusion to inhibit proliferation of bacterial pathogens, and a vacuolar-collapse system to limit the spread of systemically viral pathogens.
VPE-dependent susceptible cell death
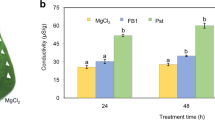
VPE-dependent cell death is also involved in the fungal toxin-induced susceptible cell death of Arabidopsis thaliana.24 Toxin-induced cell death is a strategy of pathogens for infection. Some compatible pathogens secrete toxins to kill host cells and trigger lesion formation at the infection site. Fungal toxins have been shown to induce PCD in animal and plant cells.44 A host-selective mycotoxin, fumonisin B1 (FB1), which is produced by fungal pathogens, forms lesions on Arabidopsis leaves and induces collapse of the vacuolar membrane.24 An Arabidopsis VPE-null mutant, lacking all four VPE genes, exhibits neither lesion formation nor vacuolar collapse in the leaves after FB1 infiltration. It should be noted that VPE mediates both hypersensitive cell death as a plant defense strategy, and toxin-induced cell death as a pathogen strategy. During evolution, pathogens might have overcome the plant defense response by utilizing a VPE-dependent cell death system in plants.
VPE-dependent developmental cell death
The Arabidopsis genome has four VPE homologues: αVPE, βVPE, γVPE, and δVPE. The vegetative-types, αVPE and γVPE, are upregulated during tracheary element differentiation, during leaf senescence, and after treatment with salicylic acid.7, 45 Accordingly, VPE is involved in both pathogen-induced cell death and developmental cell death.
Arabidopsis δVPE is specifically and transiently expressed in two cell layers of the seed coat (ii2 and ii3) at an early stage of seed development.25 At this stage, cell death accompanying vacuolar collapse occurs in the ii2 layer, followed by cell death in the ii3 layer (Figure 3). In a δVPE-deficient mutant, cell death of the two layers of the seed coat was delayed. Immunocytochemical analysis localized δVPE to electron-dense structures inside and outside the walls of seed coat cells undergoing cell death. At the early stage of seed development, δVPE is involved in the cell death of limited cell layers; the purpose of this process is to form a seed coat.25
VPE-dependent vacuole-mediated cell death for development of seed coats. At an early stage of seed development, cell death accompanying vacuolar collapse and cell shrinkage occurs in the ii2 layer, followed by cell death in the ii3 layer. δVPE is involved in the cell death of the limited cell layers, the purpose of which is to form a hard seed coat
Vacuolar collapse has also been shown to trigger degradation of the cytoplasmic structures during the differentiation of tracheary elements, leading to cell death.46 VPE-dependent vacuolar cell death, which degrades dying cells, is useful in plants, because plant cells surrounded with rigid cell walls must degrade materials internally.
Involvement of caspase-1-like activity
As is caspase-3-like activity (described above), caspase-1-like activity is also involved in various types of plant PCDs including the above described VPE-dependent cell death. The Arabidopsis VPE-null mutant (αvpe βvpe γvpe δvpe) has been shown to lack YVADase activity,24 and the VPE activity has been shown to be nicely correlated with the YVADase activity in tobacco VPE-silenced plants.23 Accordingly, VPE was identified as the proteinase responsible for caspase-1-like activity in Arabidopsis and tobacco plants. Although VPE is not related to the caspase family or the metacaspase family,47 VPE and caspase-1 share several enzymatic properties of the catalytic dyad, the active site, and substrate Asp pocket.48, 49, 50 Both VPE49, 51, 52 and caspase-148 are subject to self-catalytic conversion/activation from their inactive precursors. A key difference between VPE and caspase-1 is that VPE is localized in vacuoles, unlike caspases, which are localized in the cytosol. Although plants and animals both rely on YVADase activity for cell death, VPE-dependent cell death differs from caspase-1-dependent animal cell death.
Vacuolar processing enzyme/asparagine endopeptidase (VPE/AEP)
Vacuolar collapse is initiated by a vacuolar enzyme, VPE,22, 23, 24, 25, 43 which was originally identified as a processing enzyme responsible for the maturation of seed storage proteins in protein storage vacuoles.5, 53 Studies indicate that plant VPE is responsible for the maturation or activation of vacuolar proteins.4, 5, 6, 7, 8, 54, 55, 56 In developing pumpkin seeds, VPE functions in the production of multifunctional proteins, including cytotoxic peptides and trypsin inhibitors, that may act in defense by processing a larger precursor protein, PV100.15 Although VPE is a proteolytic enzyme, it also functions as a transpeptidase to make a new peptide bond, in association with cleaving a peptide bond at the C-terminal side of asparagine or aspartic acid residue.57
VPE is widely distributed in plants and animals and is also referred as to AEP (asparagine endopeptidase) in animals. Analyses with AEP-null mice have shown that AEP is required for the maturation of lysosomal proteinases (cathepsins B, L, and H), and that AEP has a critical role in the endosomal/lysosomal degradation of kidney cells.58 Recently, it was reported that APE is involved in neuronal cell death, whereby AEP appears to degrade a DNase inhibitor (SET), which is a caspase substrate, and trigger DNA damage in the brain.59 A similar VPE/AEP-dependent mechanism may function in animal PCD.
Conclusions
Using vacuoles especially for defense cell death makes sense for plants, because plants lack immune cells and so, each cell has to provide its own defense against pathogens. Plants use the vacuoles depending on the type of pathogenic organism involved. Membrane fusion, which is normally suppressed, was triggered in a proteasome-dependent manner by the bacterial infection. However, the membrane fusion system does not work on viruses, because most of defense proteins do not stop viral propagation. The membrane fusion strategy provides plants with a mechanism for attacking extracellular bacterial pathogens, and complements a vacuolar-collapse strategy. Plants have evolved two types of vacuole-mediated cell death as the cell-autonomous immune system, a membrane fusion-mediated system to kill bacterial pathogens, and a vacuolar collapse system to stop viral proliferation. The latter system, which causes rapid degradation of cellular materials, can be used not only for differentiation of cells and tissues in plants, but also for non-apoptotic cell death in animals.
Abbreviations
- VPE:
-
vacuolar processing enzyme
- PR proteins:
-
pathogenesis-related proteins
- HR:
-
hypersensitive response
- PCD:
-
programmed cell death
References
Jauh GY, Phillips TE, Rogers JC . Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 1999; 11: 1867–1882.
Shimada T, Koumoto Y, Li L, Yamazaki M, Kondo M, Nishimura M et al. AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol 2006; 47: 1187–1194.
Yamazaki M, Shimada T, Takahashi H, Tamura K, Kondo M, Nishimura M et al. Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol 2008; 49: 142–156.
Hara-Nishimura I, Inoue K, Nishimura M . A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett 1991; 294: 89–93.
Hara-Nishimura I, Takeuchi Y, Nishimura M . Molecular characterization of a vacuolar processing enzyme related to a putative cysteine proteinase of Schistosoma mansoni. Plant Cell 1993; 5: 1651–1659.
Hara-Nishimura I, Shimada T, Hiraiwa N, Nishimura M . Vacuolar processing enzyme responsible for maturation of seed proteins. J Plant Physiol 1995; 145: 632–640.
Hara-Nishimura I, Maeshima M . Vacuolar processing enzymes and auqaporins. In: Robinson ADG, Rogers JC (eds). Vacuolar Compartments in Plants. Sheffield Academic Press: London, UK, 2000, pp 20–42.
Yamada K, Shimada T, Nishimura M, Hara-Nishimura I . A VPE family supporting various vacuolar functions in plants. Physiol Plant, Special Issue 2005; 123: 369–375.
Hiraiwa N, Kondo M, Nishimura M, Hara-Nishimura I . An aspartic proteinase is involved in the maturation of storage proteins in concert with the vacuolar processing enzyme. Eur J Biochem 1997; 246: 133–141.
Yamada K, Matsushima R, Nishimura M, Hara-Nishimura I . A slow maturation of a cysteine protease with a granulin domain in the vacuoles of senescing Arabidopsis leaves. Plant Physiol 2001; 127: 1626–1634.
Minami A, Fukuda H . Transient and specific expression of a cysteine endopeptidase associated with autolysis during differentiation of Zinnia mesophyll cells into tracheary elements. Plant Cell Physiol 1995; 36: 1599–1606.
Obara K, Kuriyama H, Fukuda H . Direct evidence of active and rapid nuclear degradation triggered by vacuole rupture during programmed cell death in zinnia. Plant Physiol 2001; 125: 615–626.
Neuhaus J-M, Sticher L, Meins FJ, Boller T . A short C-terminal sequence is necessary and sufficient for the targeting of chitinase to the plant vacuole. Proc Natl Acad Sci USA 1991; 88: 10362–10366.
Ueda H, Nishiyama C, Shimada T, Koumoto Y, Hayashi Y, Kondo M et al. AtVAM3 is required for normal specification of idioblasts, myrosin cells. Plant Cell Physiol 2006; 47: 164–175.
Yamada K, Shimada T, Kondo M, Nishimura M, Hara-Nishimura I . Multiple functional proteins are produced by cleaving Asn-Gln bonds of a single precursor by vacuolar processing enzyme. J Biol Chem 1999; 274: 2563–2570.
Bowles DJ, Marcus SE, Pappin DJC, Findlay JBC, Eliopoulos E, Maycox PR et al. Posttranslational processing of concanavalin A precursors in jackbean cotyledons. J Cell Biol 1986; 102: 1284–1297.
Bednarek SY, Raikhel NV . The barley lectin carboxy-terminal propeptide is a vacuolar protein sorting determinant in plants. Plant Cell 1991; 3: 1195–1206.
Jones JD, Dangl JL . The plant immune system. Nature 2006; 444: 323–329.
Goodman RN, Novacky AJ . The Hypersensitive Response Reaction in Plants to Pathogens: A Resistance Phenomenon. American Phytopathological Society Press: St. Paul, MN, 1994.
Greenberg JT . Programmed cell death in plant-pathogen interaction. Annu Rev Plant Physiol Plant Mol Biol 1997; 48: 525–545.
Dangl JL, Jones JD . Plant pathogens and integrated defence responses to infection. Nature 2001; 411: 826–833.
Hara-Nishimura I, Hatsugai N, Kuroyanagi M, Nakaune S, Nishimura M . Vacuolar processing enzyme: an executor of plant cell death. Curr Opinon Plant Biol 2005; 8: 404–408.
Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M et al. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 2004; 305: 855–858.
Kuroyanagi M, Yamada K, Hatsugai N, Kondo M, Nishimura M, Hara-Nishimura I . Vacuolar processing enzyme is essential for mycotoxin-induced cell death in Arabidopsis thaliana. J Biol Chem 2005; 280: 32914–32920.
Nakaune S, Yamada K, Kondo M, Kato T, Tabata S, Nishimura M et al. A vacuolar processing enzyme, dVPE, is involved in seed coat formation at the early stage of seed development. Plant Cell 2005; 17: 876–887.
Kuriyama H, Fukuda H . Developmental programmed cell death in plants. Curr Opin Plant Biol 2002; 5: 568–573.
Hatsugai N, Iwasaki S, Tamura K, Kondo M, Fuji K, Ogasawara K et al. A novel membrane-fusion-mediated plant immunity against bacterial pathogens. Gene Dev 2009; 23: 2496–2506.
Mackey D, Holt III BF, Wiig A, Dangl JL . RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 2002; 108: 743–754.
Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL . Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 2003; 112: 379–389.
Axtell MJ, Staskawicz BJ . Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 2003; 112: 369–377.
van Loon LC, Rep M, Pieterse CM . Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 2006; 44: 135–162.
Dreher K, Callis J . Ubiquitin, hormones and biotic stress in plants. Ann Bot (Lond) 2007; 99: 787–822.
Craig A, Ewan R, Mesmar J, Gudipati V, Sadanandom A . E3 ubiquitin ligases and plant innate immunity. J Exp Bot 2009; 60: 1123–1132.
Lam E, del Pozo O . Caspase-like protease involvement in the control of plant cell death. Plant Mol Biol 2000; 44: 417–428.
Woltering EJ . Death proteases come alive. Trends Plant Sci 2004; 9: 469–472.
Bonneau L, Ge Y, Drury GE, Gallois P . What happened to plant caspases? J Exp Bot 2008; 59: 491–499.
Smalle J, Vierstra RD . The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol 2004; 55: 555–590.
Kurepa J, Smalle JA . Structure, function and regulation of plant proteasomes. Biochimie 2008; 90: 324–335.
Yang P, Fu H, Walker J, Papa CM, Smalle J, Ju YM et al. Purification of the Arabidopsis 26 S proteasome: biochemical and molecular analyses revealed the presence of multiple isoforms. J Biol Chem 2004; 279: 6401–6413.
Brown DG, Sun XM, Cohen GM . Dexamethasone-induced apoptosis involves cleavage of DNA to large fragments prior to internucleosomal fragmentation. J Biol Chem 1993; 268: 3037–3039.
Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C . Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol 1996; 6: 427–437.
Mittler R, Simon L, Lam E . Pathogen-induced programmed cell death in tobacco. J Cell Sci 1997; 110: 1333–1344.
Hatsugai N, Kuroyanagi M, Nishimura M, Hara-Nishimura I . A cellular suicide strategy of plants: vacuole-mediated cell death. Apoptosis 2006; 11: 905–911.
Gilchrist DG . Programmed cell death in plant disease: the purpose and promise of cellular suicide. Annu Rev Phytopathol 1998; 36: 393–414.
Kinoshita T, Yamada K, Hiraiwa N, Nishimura M, Hara-Nishimura I . Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions. Plant J 1999; 19: 43–53.
Fukuda H . Xylogenesis: initiation, progression, and cell death. Annu Rev Plant Physiol Plant Mol Biol 1996; 47: 299–325.
Uren AG, Orourke K, Aravind L, Pisabarro TM, Seshagiri S, Koonin EV et al. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT Lymphoma. Mol Cell 2000; 6: 961–967.
Cohen GM . Caspase: the executioners of apoptosis. Biochem J 1997; 326: 1–16.
Hiraiwa N, Nishimura M, Hara-Nishimura I . Vacuolar processing enzyme is self-catalytically activated by sequential removal of the C-terminal and N-terminal propeptides. FEBS Lett 1999; 447: 213–216.
Wilson KP, Black J-AF, Thomson JA, Kim EE, Griffith JP, Navia MA et al. Structure and mechanism of interleukin-1 beta converting enzyme. Nature 1994; 370: 270–274.
Hiraiwa N, Nishimura M, Hara-Nishimura I . Expression and activation of the vacuolar processing enzyme in Saccharomyces cerevisiae. Plant J 1997; 12: 819–829.
Kuroyanagi M, Nishimura M, Hara-Nishimura I . Activation of Arabidopsis vacuolar processing enzyme by self-catalytic removal of an auto-inhibitory domain of the C-terminal propeptide. Plant Cell Physiol 2002; 43: 143–151.
Hara-Nishimura I, Nishimura M . Proglobulin processing enzyme in vacuoles isolated from developing pumpkin cotyledons. Plant Physiol 1987; 85: 440–445.
Hiraiwa N, Takeuchi Y, Nishimura M, Hara-Nishimura I . A vacuolar processing enzyme in maturing and germinating seeds: its distribution and associated changes during development. Plant Cell Physiol 1993; 34: 1197–1204.
Mitsuhashi N, Hayashi Y, Koumoto Y, Shimada T, Fukasawa-Akada T, Nishimura M et al. A novel membrane protein that is transported to protein-storage vacuoles via precursor-accumulating vesicles. Plant Cell 2001; 13: 2361–2372.
Shimada T, Yamada K, Kataoka M, Nakaune S, Koumoto Y, Kuroyanagi M et al. Vacuolar processing enzymes are essential for proper processing of seed storage proteins in Arabidopsis thaliana. J Biol Chem 2003; 278: 32292–32299.
Saska I, Gillon AD, Hatsugai N, Dietzgen RG, Hara-Nishimura I, Anderson MA et al. An asparaginyl endopeptidase mediates in vivo protein backbone cyclization. J Biol Chem 2007; 282: 29721–29728.
Shirahama-Noda K, Yamamoto A, Sugihara K, Hashimoto N, Asano M, Nishimura M et al. Biosynthetic processing of cathepsins and lysosomal degradation are abolished in asparaginyl endopeptidase-deficient mice. J Biol Chem 2003; 278: 33194–33199.
Liu Z, Jang S-W, Liu X, Cheng D, Peng J, Yepes M et al. Neuroprotective actions of PIKE-L by inhibition of SET proteolytic degradation by asparagine endopeptidase. Mol Cell 2008; 29: 665–678.
Acknowledgements
We are grateful to the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) for Grants-in-Aid for Scientific Research (no. 22000014) and for the Global Center of Excellence Program ‘Formation of a Strategic Base for Biodiversity and Evolutionary Research: from Genome to Ecosystem’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by P Bozhkov
Rights and permissions
About this article
Cite this article
Hara-Nishimura, I., Hatsugai, N. The role of vacuole in plant cell death. Cell Death Differ 18, 1298–1304 (2011). https://doi.org/10.1038/cdd.2011.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2011.70
Keywords
This article is cited by
-
The role of melatonin on caspase-3-like activity and expression of the genes involved in programmed cell death (PCD) induced by in vitro salt stress in alfalfa (Medicago sativa L.) roots
Botanical Studies (2022)
-
Plant-derived extracellular vesicles: a novel nanomedicine approach with advantages and challenges
Cell Communication and Signaling (2022)
-
Microgravity enhances the phenotype of Arabidopsis zigzag-1 and reduces the Wortmannin-induced vacuole fusion in root cells
npj Microgravity (2022)
-
Characterization and fine mapping of a lesion mimic mutant (Lm5) with enhanced stripe rust and powdery mildew resistance in bread wheat (Triticum aestivum L.)
Theoretical and Applied Genetics (2022)
-
The Rep and C1 of Beet curly top Iran virus represent pathogenicity factors and induce hypersensitive response in Nicotiana benthamiana plants
Virus Genes (2022)